Electroreduction of nitrogen with almost 100% current-to-ammonia efficiency - Nature
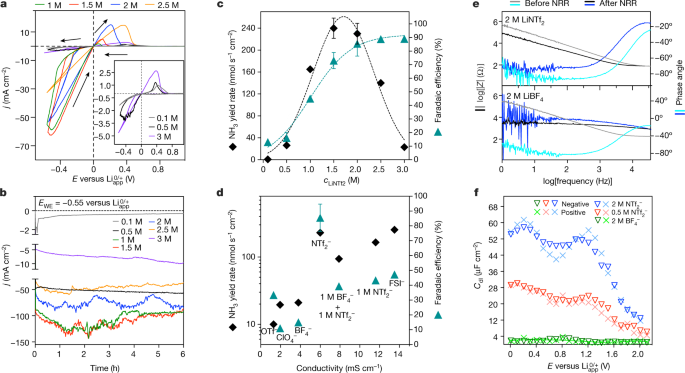
A high-efficiency, robust process using a high-concentration imide-based lithium-salt electrolyte enables the electroreduction of nitrogen with stabilized ammonia yield rates of 150 ± 20 nmol s−1 cm−2 and a current-to-ammonia efficiency that is close to 100%.
I am usually skeptical to the point of not reading 'new and exciting developments' but this one caught my eye, mostly because it is published in Nature, and because Ammonia has come up over the years as a possibly viable liquid fuel for energy storage
I'd love to hear technical discussion to fill in my knowledge gaps
Scalability ?
Cost ?
Transport ?
Combustion ? Oxidation via Fuel Cell ?
Nitrogen recovery ?