NB: This discussion started in another thread and in another sub, but I felt we were getting far afield from the thread topic. Since I contributed to that, I took it on myself to start a new thread. Anyway I've been wanting a dedicated thread for power-to-methane discussion.
The story so far:
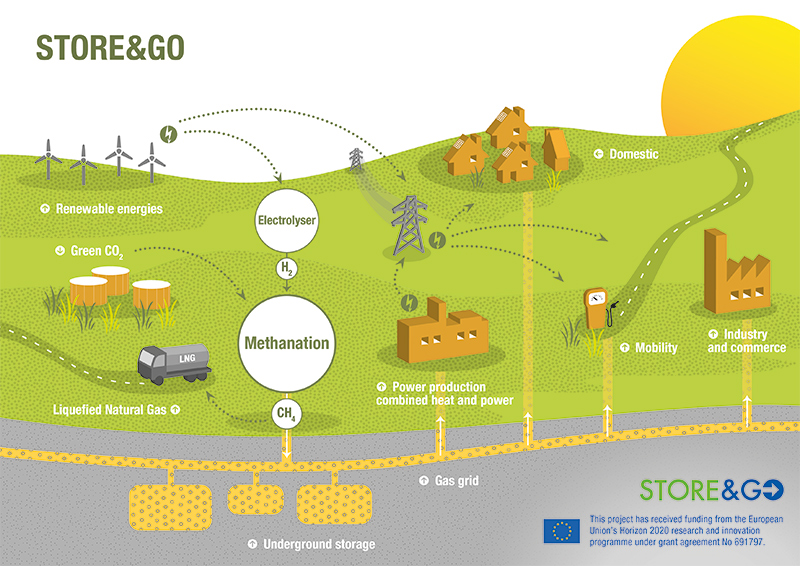
My understanding from reading about projects like HELMETH is that the process requires a fairly pure stream of CO2 — at least with the current state of the art, and for an end product that's pure enough to use in natural gas infrastructure. Do you know of a sufficiently efficient method for direct air capture of CO2?
On the topic of efficiency, HELMETH claimed about 75% in their pilot, and suggested that 80% would be practical at scale. As I understand it they got to that point primarily by using excess heat from one stage in the process to boost another stage.
The story so far:
So my outsider view of things - it looks like we've got much more widespread systems for managing liquid methane (roughly the same as natural gas) than we do hydrogen, it's less dangerous to be working with at all - so why not be using the excess renewable energy to manufacture liquid methane than hydrogen?
I also like the idea of turning excess renewable energy into CH4, so we can use all the existing LNG and natural gas infrastructure. However methane is a powerful greenhouse gas itself, so we have to be careful about leaks. Also combustion of methane produces CO2; we have to find ways to capture that CO2 and feed it back into methane synthesis. That may be easy in some applications: imagine an efficient gas turbine co-located with CO2 storage and a methane synthesis plant. But capturing the CO2 from an oceangoing freight vessel sounds more difficult.
Controlling methane leaks is something to consider (since it's harder to extract from air), but the CO2 emissions isn't. There's nothing to be gained from storing the CO2 in a container versus storing it in the atmosphere and re-extracting it later for the sabatier process [for converting to methane].
So not a concern. The problem holding this idea back is that it takes much more energy to produce methane versus H2, and you don't get as much back burning methane versus running H2 through a fuel cell stack.
My understanding from reading about projects like HELMETH is that the process requires a fairly pure stream of CO2 — at least with the current state of the art, and for an end product that's pure enough to use in natural gas infrastructure. Do you know of a sufficiently efficient method for direct air capture of CO2?
On the topic of efficiency, HELMETH claimed about 75% in their pilot, and suggested that 80% would be practical at scale. As I understand it they got to that point primarily by using excess heat from one stage in the process to boost another stage.