By using silicon nanowires, the capacity of Li-ion batteries could be greatly boosted. Something to watch.
Welcome to Tesla Motors Club
Discuss Tesla's Model S, Model 3, Model X, Model Y, Cybertruck, Roadster and More.
Register
Install the app
How to install the app on iOS
You can install our site as a web app on your iOS device by utilizing the Add to Home Screen feature in Safari. Please see this thread for more details on this.
Note: This feature may not be available in some browsers.
-
Want to remove ads? Register an account and login to see fewer ads, and become a Supporting Member to remove almost all ads.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
tonybelding
Active Member
By using silicon nanowires, the capacity of Li-ion batteries could be greatly boosted. Something to watch.
For convenience, here's a repeat of the same info I posted in the other thread (PHEVs Moving In). . .
Some researchers contacted by Chemistry World questioned whether the technique would be useful for commercial batteries. 'The most appealing result is obviously the high cycling capacity that these materials are able to deliver,' said one leading expert on lithium battery anodes, who asked not to be named. 'However, the test is limited to only 10 cycles and this is far too few to determine the industrial impact of the electrode. Also, the rate of the cycling test is very low and thus the power capability, another important practical requisite, has not been ascertained.'
Full article here: http://www.rsc.org/chemistryworld/News/2007/December/17120702.asp
Also this. . .
"It's a really nice proof of concept," says Gerbrand Ceder, a materials scientist and battery expert at the Massachusetts Institute of Technology in Cambridge. Making lithium ion batteries capable of holding 10 times the charge of conventional versions still requires a cathode that holds 10 times the charge, too, Ceder says. However, he adds, incorporating a silicon nanowire-based anode could allow batterymakers to reduce the weight and volume of the anode and add more cathode material in its place, which could give lithium batteries a power boost.
Full article here: http://sciencenow.sciencemag.org/cgi/content/full/2007/1217/2
That is an exciting development. The nanotechnology is reminiscent of the ultracap breakthrough that MIT had previously announced (but has yet to show up commercially). Using nanotech like that for Li-Ion would make it much easier for Tesla since the voltage characteristics of batteries are easier to deal with than the behavior of ultracapacitors.
Still, a lab breakthrough doesn't always mean production ready anytime soon (if ever).
Still, a lab breakthrough doesn't always mean production ready anytime soon (if ever).
JRP3
Hyperactive Member
I see this as more of an advancement in durability and cycle life than capacity, and I don't know if it's any improvement over what Altairnano has already achieved.
JRP3
Hyperactive Member
Another report, don't hold your breath on this one:
Technology Review: Super-Charging Lithium BatteriesThe downside is that the nanowire growth process that Cui uses, which feeds gaseous silicon to a liquid gold catalyst to make the solid electrode, is a high-temperature (600 to 900 °C) process that could be costly to scale up. Cui believes that scale-up of the vapor-liquid-solid process is nevertheless feasible, but he acknowledges that he is also "exploring another approach."
Ohio State University chemist Yiying Wu, who also works on nanowire electrodes, calls the Stanford work "definitely very important." But Wu and other materials scientists caution that additional advances will be required before lithium batteries with nanowire electrodes deliver major increases in performance of electric-vehicle batteries. Not least is the need to scale up the process of making nanowires, which have yet to be mass-produced for commercial application.
Another limitation is that while Cui's silicon nanowires make great anodes, lithium-battery technology has greater need for improved cathodes. In a given battery, substituting an anode that stores more lithium ions has no impact without a corresponding cathode that can supply more charge.
Bit of a blast from the past, but if you haven't seen it:
High-performance lithium battery anodes using silicon nanowires : Article : Nature Nanotechnology
or if you prefer
http://www.nature.com/nnano/journal/v3/n1/pdf/nnano.2007.411.pdf
High-performance lithium battery anodes using silicon nanowires : Article : Nature Nanotechnology
or if you prefer
http://www.nature.com/nnano/journal/v3/n1/pdf/nnano.2007.411.pdf
Serge
Member
It seems cycle life is the problem with silicon nanowire anodes, but there is progress. Apparently they got the cycle count up to 200 at 20% capacity loss.
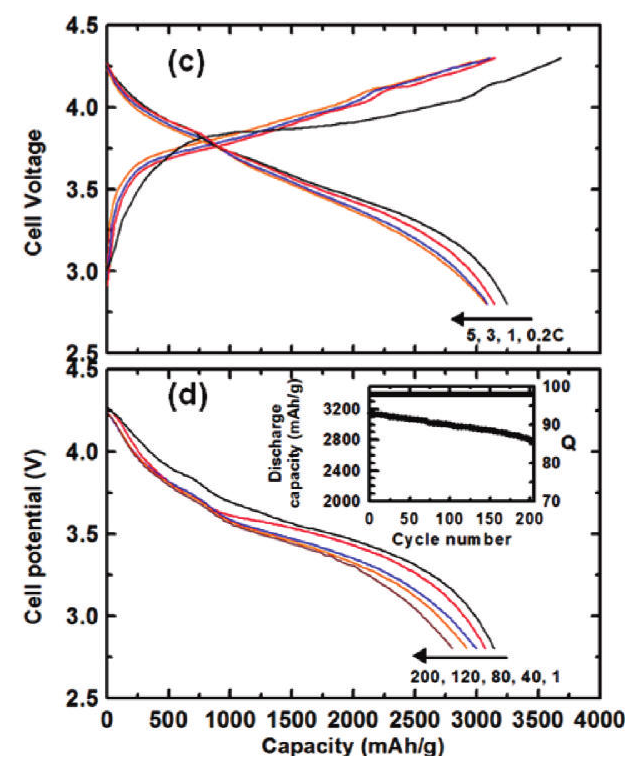
Prepared by reductive decomposition of a silicon precursor in an alumina template and etching, the Si nanotubes show a very high reversible charge capacity of 3,247 mAh/g with Coulombic efficiency of 89%, and also demonstrate a superior capacity retention even at a 5C rate (=15 A/g). A Li-ion full cell consisting of a LiCoO2 cathode and Si nanotube anode demonstrated 10 times higher capacity than commercially available cells with graphite anodes even after 200 cycles.
JRP3
Hyperactive Member
I'm not sure how, but it looks like the loss is logarithmic, the amount of capacity lost between 120 to 200 cycles is about equal to the amount between 80-120 and between 40-80 cycles. If this continues, it would lose the same amount from 200 to 320 cycles.
This battery would still be an advance if partial cycles scale (i.e. two half charges = 1 full cycle)... a battery half the weight of Tesla's current design would only charge 20% per day, so after 1000 cycles it would have lost 20% of its full charge capacity, and still would last 4 times longer than the current battery!
This battery would still be an advance if partial cycles scale (i.e. two half charges = 1 full cycle)... a battery half the weight of Tesla's current design would only charge 20% per day, so after 1000 cycles it would have lost 20% of its full charge capacity, and still would last 4 times longer than the current battery!
Serge
Member
Amprius
Cui's technology moving from lab to manufacturing
Interestinly, Tesla is part of the team.
Cui's technology moving from lab to manufacturing
Amprius is in talks with vehicle and electronics manufacturers, and raised its first round of venture funding in March. The company hopes to raise more funds next summer to build a pilot manufacturing line.
No matter how good the anode is, the overall charge capacity of a battery depends on the cathode, too. The performance of today's lithium-ion cathodes isn't as good as that of the anodes Amprius is developing. The company's initial battery designs make up for this mismatch by pairing a thin anode with a thick cathode. Compared to a conventional lithium-ion battery of equal size, this design stores 40 percent more charge. In order to further increase the energy density, however, the company will need new cathode materials
Interestinly, Tesla is part of the team.
Interestinly, Tesla is part of the team.
Actually, it doesn't say that Tesla is part of the team. It says:
Which sounds to me more like they've hired former Tesla engineers, not done a deal with the company.Key players from the team that developed and manufactured the only production lithium-ion battery pack available for vehicles at Tesla Motors
Serge
Member
Indeed. However why then is Tesla Motors corporate logo displayed so prominently?Actually, it doesn't say that Tesla is part of the team. It says:
Which sounds to me more like they've hired former Tesla engineers, not done a deal with the company.
JRP3
Hyperactive Member
JRP3
Hyperactive Member
Glad I didn'tdon't hold your breath on this one
Dr. J
Active Member
Yeah, that would've gotten uncomfortable after a year or two, for sure.Glad I didn't
Similar threads
- Replies
- 12
- Views
- 921
- Replies
- 0
- Views
- 74
- Replies
- 2
- Views
- 470
- Replies
- 8
- Views
- 172



